Unmet Need
Tolerability Challenges Unnecessary Exposure to
Extended Endocrine Therapy Side Effects Lead
to Noncompliance
In one test, BCI combines two complementary gene-expression signatures to assess proliferative and estrogen signaling pathways 1 - 3
Predictive Score
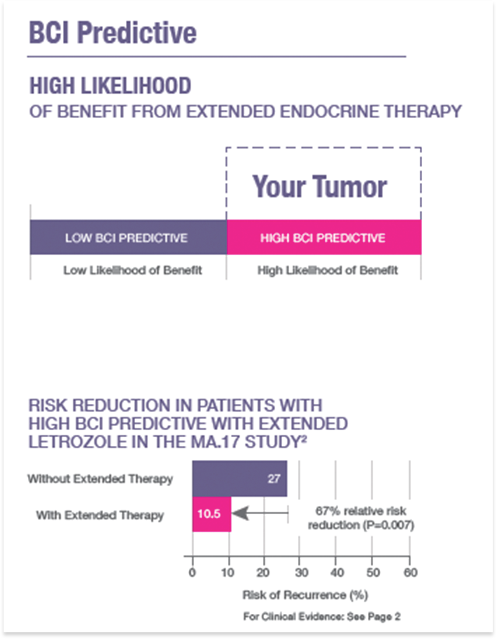
Reports the individualized likelihood of benefit from extended endocrine therapy
- Quantitative molecular assessment of estrogen signaling pathways
- Genes: HoxB13/IL17BR (H/I)
- Binary result (HIGH/LOW)
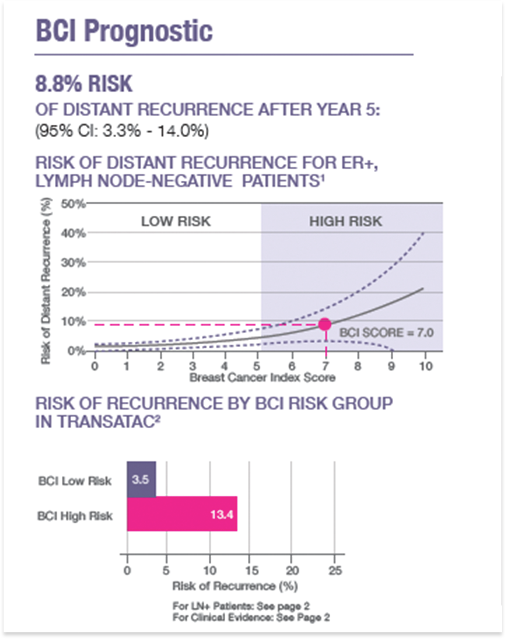
Prognostic Score
Reports the individualized risk of late distant recurrence of breast cancer (Years 5 – 10)2,3
- Algorithmic combination of Molecular Grade Index (MGI)
- Genes: BUB1B, CENPA, NEK2, RACGAP1, RRM2, H/I
- Numerical result reported on a continuous curve (delineated by HIGH/LOW risk categories)
For all patients, clinical decisions require incorporation of BCI results with all other clinical and pathological risk factors, patient tolerability, and comorbidities *For node-negative patients. Node positive patients should be considered to have a high risk of recurrence Risk = Prognostic Risk of Recurrence in Years 5-10; Benefit = Predicted Benefit from Extended Endocrine Therapy
References
- Ma X-J, et al. Clin Cancer Res. 2008;14:2601-2608.
- Sgroi DC, et al. Lancet Oncol. 2013;14:1067 – 76.
- Zhang Y, et al. Clin Cancer Res. 2013;19:4196-4205.
- Goss PE, et al. N Engl J Med. 2003;349:1793-1802.
- Burstein HJ, et al. J Clin Oncol. 2014;32:1-16
Breast Cancer Index Indications for Use and Limitations
BCI provides a quantitative assessment of the likelihood of distant recurrence in patients diagnosed with ER+ node-negative breast cancer, and prediction of likelihood of benefit from extended (>5 year) endocrine therapy in patients who are recurrence-free after an initial 5 years of adjuvant endocrine therapy. Treatment decisions require correlation with all other clinical findings. This test was developed and its performance characteristics determined by bioTheranostics, Inc. lt has not been cleared or approved by the U.S. Food and Drug Administration. This test is used for clinical purposes. lt should not be regarded as investigational or for research. How this information is used to guide patient care is the responsibility of the physician. bioTheranostics is certified under the Clinical Laboratory lmprovement Amendments of 1988 to perform high-complexity clinical laboratory testing.