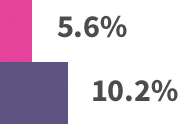
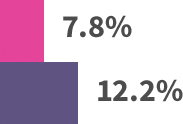
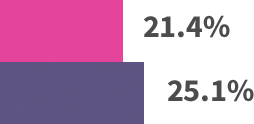
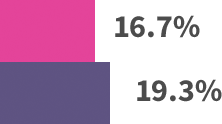
Most patients on extended endocrine therapy are unnecessarily exposed to risks and side effects.
Across 4 major trials, only about
3 – 5% of patients
benefit from extended endocrine therapy.1,2,5-7
Better patient selection is needed to target patients who would benefit from extended endocrine therapy.
Modest patient benefit across trials:*
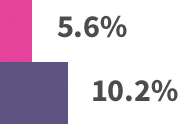
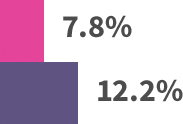
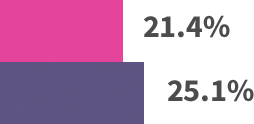
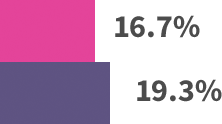
Standard predictive tools:
Current approaches do not provide the information you need for Years 5-10
Clinical and pathological factors are unable to assess likelihood of benefit from extended endocrine therapy or individualized risk of late recurrence in ER+ patients1
*Absolute benefits in extended endocrine therapy trials: MA.17 trial—4.6% absolute benefit, based on 4-year disease-free survival: 94.4% with letrozole vs 89.8% with placebo. ABCSG-6a trial—4.4% absolute benefit, based on 5-year recurrence rates: 7.8% with 3 years of anastrozole vs 12.2% with extended no treatment. ATLAS trial—3.7% absolute benefit, based on 10-year recurrence rates: 21.4% with extended tamoxifen vs 25.1% with no extended treatment. ATTom trial—2.6% absolute benefit, based on 10-year recurrence rates: 16.7% with extended tamoxifen vs 19.3% with no extended treatment.
Experts agree: establishing better predictive tools is a priority
ASCO 2014 Practice Guidelines
The need was identified for a biomarker to “selectively predict early vs. late recurrence” and “determine whether longer durations of adjuvant endocrine therapy are clinically indicated.”*
BCI provides a quantitative assessment of the likelihood of distant recurrence in patients diagnosed with ER+ node-negative breast cancer, and prediction of likelihood of benefit from extended (>5 year) endocrine therapy in patients who are recurrence-free after an initial 5 years of adjuvant endocrine therapy. Treatment decisions require correlation with all other clinical findings. This test was developed and its performance characteristics determined by bioTheranostics, Inc. lt has not been cleared or approved by the U.S. Food and Drug Administration. This test is used for clinical purposes. lt should not be regarded as investigational or for research. How this information is used to guide patient care is the responsibility of the physician. bioTheranostics is certified under the Clinical Laboratory lmprovement Amendments of 1988 to perform high-complexity clinical laboratory testing.