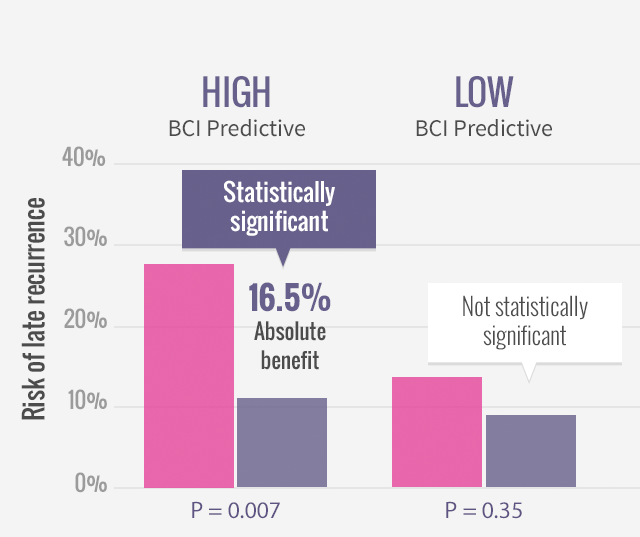
Study Design and Statistical Analysis Led by the National Cancer Institute of Canada (NCIC)3
- Biomarker study designed as a case-control study to enrich for and powered based on number of recurrences examined
- The study cohort of 249 patients included 83 recurrences, representing >30% of recurrences from the parent trial
- As a benchmark, this event rate approximates a population-based study of ~2,600 patients from the MA.17 parent trial
- Cases from patients with recurrences were matched 1:2 with cases from patients that did not recur