Unmet Need
Tolerability Challenges Unnecessary Exposure to
Extended Endocrine Therapy Side Effects Lead
to Noncompliance
Order BCI
- Avoid major safety and tolerability challenges of extended endocrine therapy
- Use BCI to target patients most likely to benefit from extended endocrine therapy
- Prescribe with confidence in the extended treatment setting and educate on the importance of compliance
- Offered in all 50 states
- CLIA-certified, CAP-accredited clinical laboratory
- bioTheranostics will coordinate sample shipment directly from hospital to lab
- Results in 7 days
- BCI uses original specimen
- Formalin-fixed, paraffin-embedded (FFPE) tissue block, or
- 3 – 4 unstained, 10µm-thick sections on glass slides (50% tumor content) and one H&E-stained slide
Breast Cancer Index Indications for Use and Limitations
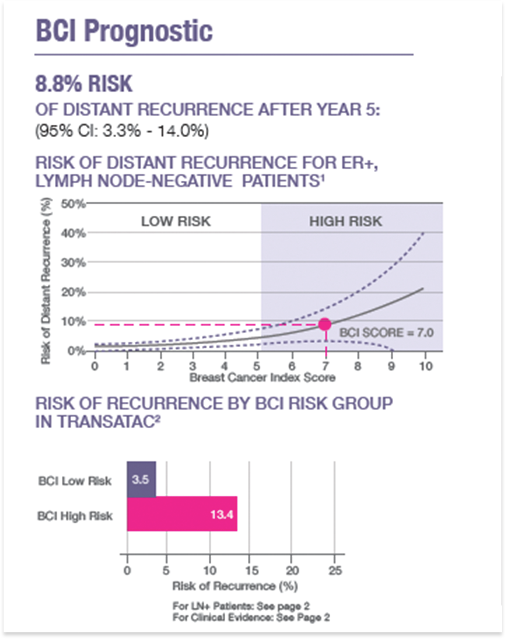
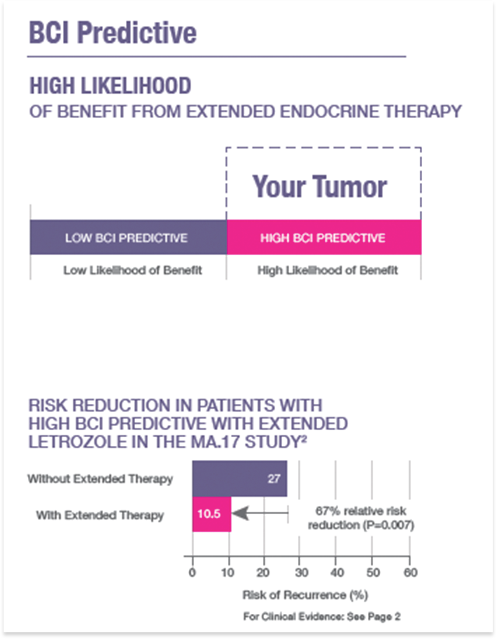
BCI provides a quantitative assessment of the likelihood of distant recurrence in patients diagnosed with ER+ node-negative breast cancer, and prediction of likelihood of benefit from extended (>5 year) endocrine therapy in patients who are recurrence-free after an initial 5 years of adjuvant endocrine therapy. Treatment decisions require correlation with all other clinical findings. This test was developed and its performance characteristics determined by bioTheranostics, Inc. lt has not been cleared or approved by the U.S. Food and Drug Administration. This test is used for clinical purposes. lt should not be regarded as investigational or for research. How this information is used to guide patient care is the responsibility of the physician. bioTheranostics is certified under the Clinical Laboratory lmprovement Amendments of 1988 to perform high-complexity clinical laboratory testing.